- About UsOpenClose
- LeadershipOpenClose
- BoardOpenClose
- TechnologyOpenClose
- PublicationsOpenClose
- PipelineOpenClose
- For PatientsOpenClose
- For InvestorsOpenClose
- NewsroomOpenClose
- Media CoverageOpenClose
- Press ReleasesOpenClose
Unlike other immune cells, our CAR-Macrophages combine a unique set of characteristics, which we believe are the key to success in solid tumor treatment:
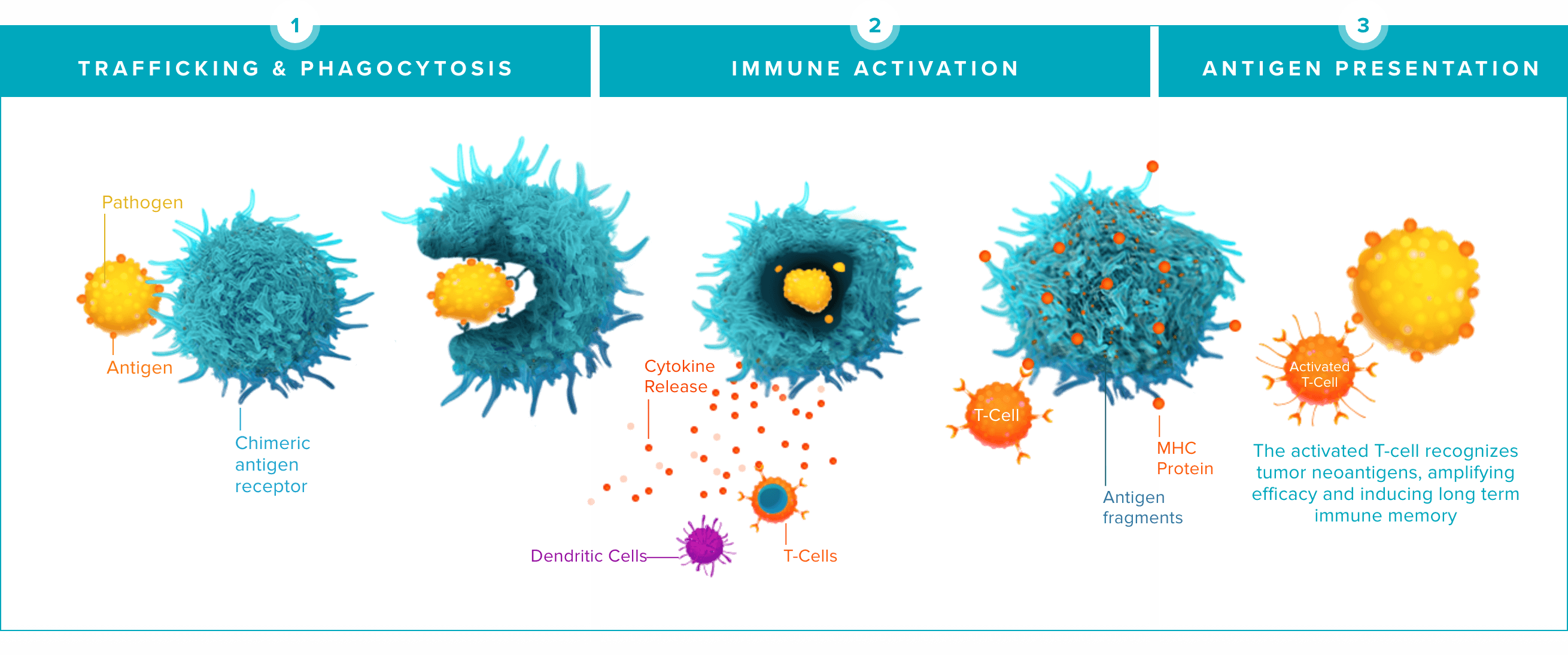
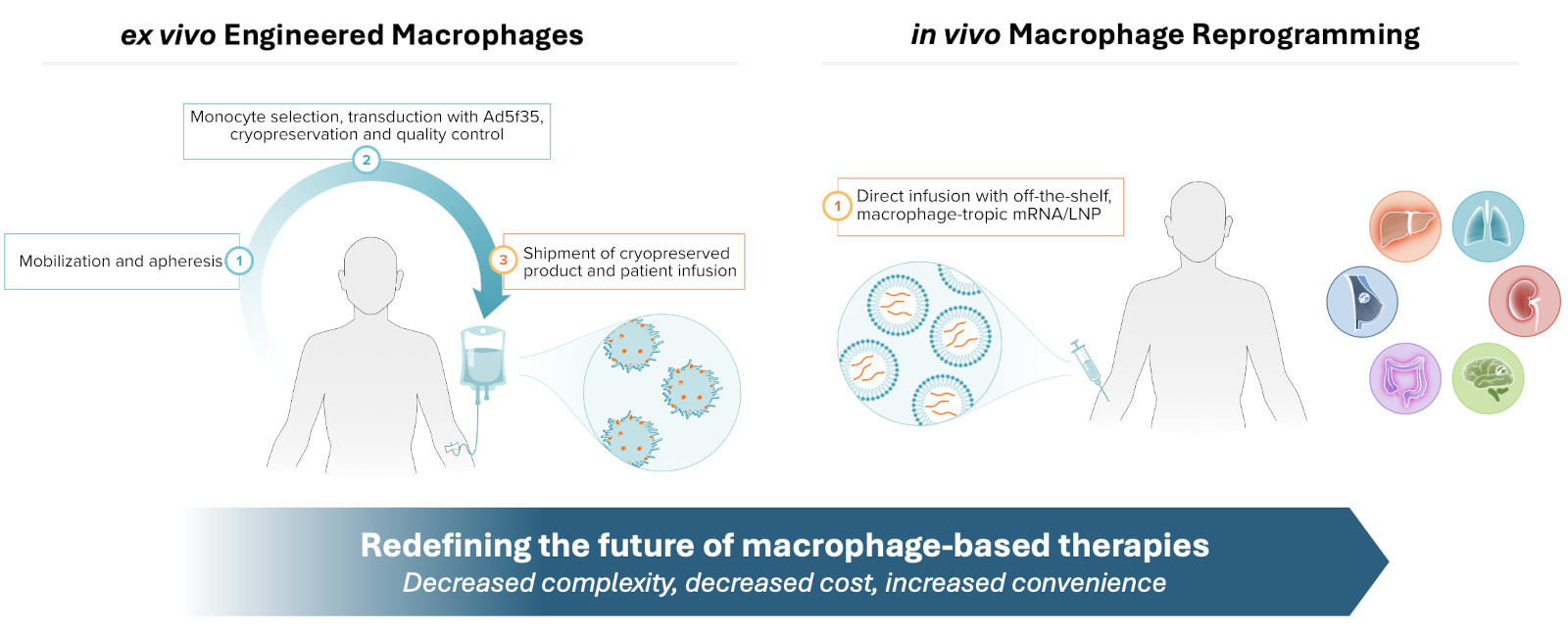
The word macrophage comes from the Greek terms “makro” meaning big and “phagein” meaning eat. Macrophages are a key player in the innate immune system, with the ability to selectively target and destroy multiple tumor cells over their lifespan. This video shows our CAR-Macrophages (in red) in action against cancer cells (in green).